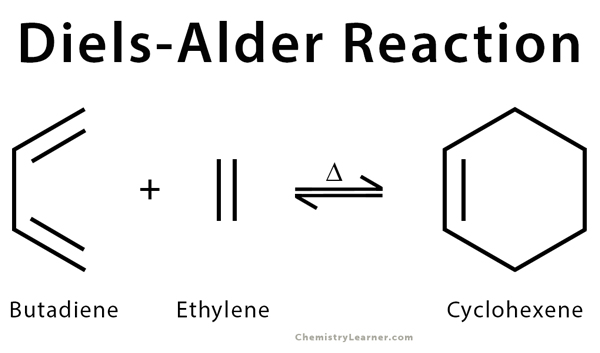
The Diels-Alder reaction is an organic reaction that is used to convert a conjugated diene (a molecule with two alternating double bonds) and a dienophile (an alkene) to a cyclic olefin. This process is concerted, where bonds form and break at the same time, and the entire reaction takes place in one step in the presence of heat. The class of reactions to which Diels-Alder belong is termed as cycloaddition. The electrons are transferred cyclically between the diene and the alkene to form a cyclic adduct. Diels-Alder reactions are stereospecific. The substituents attached to both the diene and the dienophile and retain their stereochemistry throughout the reaction. Electron withdrawing groups on the dienophile and electron-donating group on the diene facilitate reaction [1-3] .

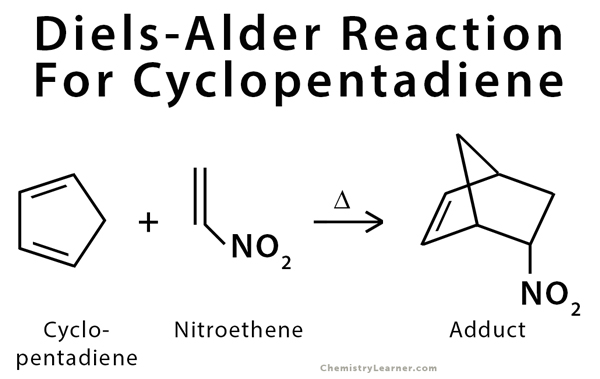
Diels-Alder Reaction Example Cyclopentadiene
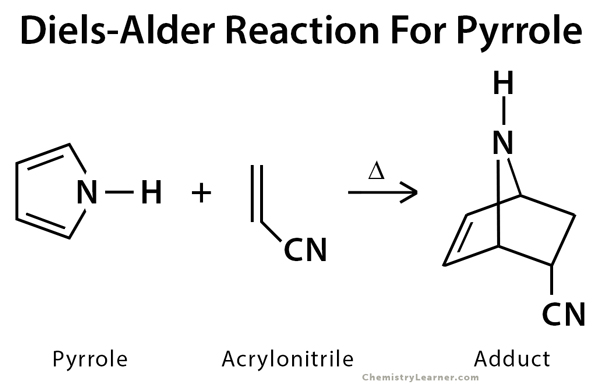
Diels-Alder Reaction Example Pyrrole
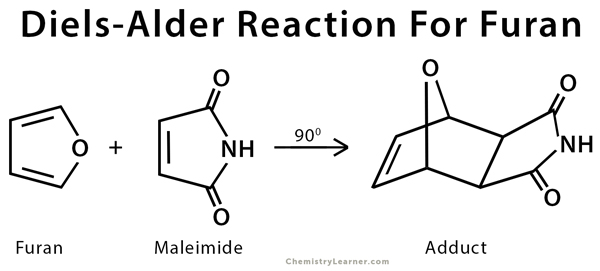
Diels Alder Reaction Example Furan
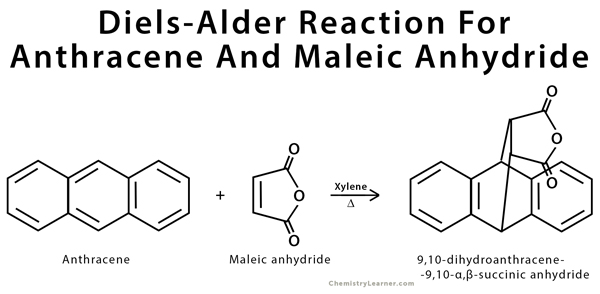
Diels-Alder Reaction Example Anthracene Maleic Anhydride
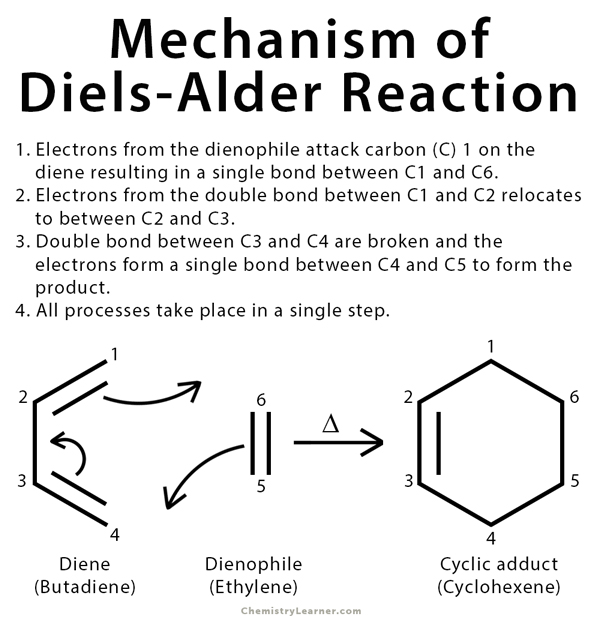
Diels-Alder Reaction Mechanism
The Diels-Alder reaction is used in the synthesis of natural products like rubber and plastic. It also finds its application in pharmaceuticals and biomedical engineering. It is used to make synthetic steroids, such as cortisone and Vitamin D [2] .
The retro Diels-Alder reaction is the exact reverse of the Diels-Alder. It passes through the same transition state when the heat is applied. For example, cyclohexene breaks down into butadiene and ethylene at a temperature of 800 °C [11-13] .

Retro Diels Alder Reaction Dicyclopentadiene